- Elkem
- Healthcare
- Pharmaceutical & drug delivery
- Drug eluting devices
Medical-grade silicones for drug-eluting devices
Medical-grade silicones are a valuable tool for drug-eluting devices, providing a permeable matrix that accommodates the API, helping drugs to be targeted where they’re needed.
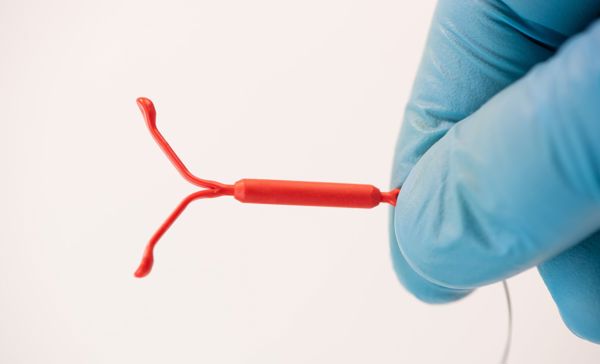
The growth of drug eluting devices is tied to several key advantages including targeting drug delivery to specific locations (lowering systemic toxicity and minimizing drug dosage) and eliminating reliance on patient compliance (through sustained release over a given time).
Biomedical-grade silicones are a great choice for drug eluting devices because silicones form a permeable matrix structure when cured, creating space for API to reside and consistently pass through over time. Silicones have also been used in implantable devices for more than 60 years.
Common examples of drug-eluting devices
- Coronary and peripheral vascular stents to reduce or prevent scar tissue formation
- Women’s Health (IUD, IVR and subcutaneous implants) for contraception, HIV prevention, or hormone replacement therapies
- Ophthalmic Devices (punctum plugs and lenses) to address dry eye or to deliver therapies to the retina
- Catheters and leads as part of larger devices to provide antimicrobial and anti-inflammatory benefits
- Pain Management
- Oncology applications for the treatment of brain tumors, prostate cancer, and bladder cancer
In addition to a comprehensive series of Silbione™ Biomedical M series Liquid Silicone Rubber (LSR) and High Consistency Rubber (HCR), which support the long-term implantable device applications, Elkem offers the Silbione™ Biomedical LSR D series that supports the drug delivery applications.
Quality and manufacturing standards
- Dedicated Elkem Silicones Quality Management System
- Elkem clean operation standard following ISO 14949 guidelines
- Certified ISO 9001 Manufacturing Facility
- Certified ISO Class 8 Manufacturing Environment
- Certified ISO Class 7 Packaging Environment
- Responsible Care Management System®
Silicone Elastomers as Modifiable Excipient for Drug Delivery Devices
This eBook is designed to explore the role of silicone as an excipient in implantable drug delivery devices.
Biocompatibility
- 12-week implant
- Hemolysis
- USP Intracutaneous Reactivity
- USP Acute Systemic Toxicity
- Cytotoxicity
- Mutagenicity Pyrogenicity
- Skin Sensitization
- Tissue Irritation
- Masterfile Support
- Drug Master File, Type IV (DMF) with FDA CDER
Our medical-grade Silbione™ products have been tested to meet USP Class VI and ISO 10993 requirements. Our drug delivery silicones have the additional testing required for long term implantation plus the submission of a Drug Master File (DMF) with the US FDA.

Experience the Silbione™ difference
Find out more about medical-grade silicones for healthcare & biomedical applications.
At Elkem Silicones, we have dedicated people located around the globe, committed to your success. Whether you are looking for a product recommendation, for customized silicone solutions, or for regulatory support, we have the people in place when and where you want them.
Silicones: A Great Choice As Excipient In Drug Delivery Systems
Learn more about Silicone properties for healthcare, Advantages of silicones in drug eluting devices and Specificities of silicone elastomer
Related applications
Contact us
Take your business to the next level by partnering with a world-leading material manufacturer.
